
Our history
“We try never to forget that medicine is for the people. It is not for the profits.”
– George Merck
For over 130 years, we've been guided by the view that great medicines and vaccines change the world.
Our legacy of inventing medicines and vaccines continues to this day. We adapt our business not only for the next quarter but for the next quarter century.
1890
1920
1930
1940
1950
1970
1980
1990
2000
2010

Founding
Merck & Co., Inc. Rahway, NJ U.S. was founded on January 1, 1891. George Merck, age 23, established the company in the U.S. to distribute fine chemicals throughout New York City and the neighbouring areas. Merck & Co., Inc., Rahway, New Jersey, U.S. is known as MSD everywhere outside of the U.S. and Canada.

The First Manual Published
Our company first published the book entitled The Merck Manual in the U.S. in 1899 (now known as The MSD Manual outside the U.S. and Canada). Treatments in the first manual included bloodletting for acute bronchitis, arsenic for impotence and almond bread for diabetes. The Manual went on to become one of the most widely used medical references in the U.S..

George W. Merck became president of our company
George Merck's son, George W. Merck, began his career in the packing and shipping department in 1914, and he received training in most branches of the business. He would lead the company through the 1927 merger with Powers-Weightman-Rosengarten Co. and turned his attention toward building research capacity, catalyzing the company’s reputation for innovation.

Created the first Research Laboratory
The MRL Research Laboratory was founded in Rahway, New Jersey. The laboratory represented our initial foray into pharmacological research and included three separate divisions: Pure Research, the Institute for Therapeutic Research and Applied Research.
Synthesized Vitamin B1
We first synthesized vitamin B₁ and published the results in the Journal of the American Chemical Society. The development allowed for mass production of the vitamin, and within a few years, the product had contributed to the reduction of vitamin B₁ deficiency (beriberi). In subsequent years, management committed the company to isolating and synthesizing vitamins and making them more widely available.


Funded the discovery of and distributed breakthrough antibiotic streptomycin
Tuberculosis was historically a leading cause of death in the U.S. In 1943, Dr. Selman Waksman and Albert Schatz discovered streptomycin, the first effective treatment for the disease. The company had supported Dr. Waksman’s research lab and held the patent rights. Once its significant health benefits were recognized, the company relinquished its patent on the antibiotic to ensure maximum patient access.
By 1950, tuberculosis-related deaths in the U.S. fell by nearly 50 percent.

We entered the animal health market with sulfaquinoxaline
After years of extensive testing, we brought S.Q. (sulfaquinoxaline) to market. The product, used toprevent coccidiosis, a parasitic poultry disease, formally ushered the company into the field of animal health.

Cortisone first commercially synthesized
Dr. Lewis Sarett, a researcher at Rahway, developed CORTONE (cortisone). The drug was used in the treatment of rheumatoid arthritis, rheumatic fever and other related chronic diseases which were often fatal and for which there was no known effective treatment.

Medicine is for the people
In a defining moment for the company, George W. Merck gave a talk at the Medical College of Virginia at Richmond, during which he made a famous statement about how the medical and pharmaceutical community could be successful:
"We try to remember that medicine is for the patient. We try never to forget that medicine is for the people. It is not for the profits. The profits follow, and if we have remembered that, they have never failed to appear."
This philosophy is embraced by our leaders and employees to this day.

Merger with Sharp & Dohme
The merger to create Merck Sharp & Dohme (MSD) [(headquartered in Rahway, New Jersey, U.S.)] brought together our extensive chemical research and manufacturing facilities with Sharp & Dohme's pharmaceutical development, marketing expertise and international presence. Sharp & Dohme's West Point, Pennsylvania facilities were included in the merger.

The Foundation was created
MSD established the company’s Foundation, a non-profit corporation dedicated to charitable giving, with an initial contribution of $500,000. To date, the Foundation has contributed hundreds of millions of dollars to non-profit organizations.

DIURIL launched to treat high blood pressure
The release of DIURIL (chlorothiazide) signaled the company's emergence as a leading cardiovascular company. Since the introduction of DIURIL, we have been at the forefront of developing new treatments to fight high blood pressure and heart disease. The “Diuril Man,” a transparent plastic figurine showing the heart, lungs, kidneys, ureters and bladder, helped MSD show physicians the value of the breakthrough product.

Treatment for trichinosis for animals discovered
In humans, trichinosis (caused by eating raw or undercooked meat) can cause high fever, muscle pain and swelling and other serious symptoms. In 1961, a research team led by Dr. William Campbell discovered thiabendazole, the first drug known to kill the trichinella parasite in sheep, goat, cattle and pigs. When the reindeer populations of the Arctic Circle became severely afflicted by parasitic infections, MSD scientists travelled north to treat them with THIBENZOLE (thiabendazole), a de-worming agent.
The treatment not only helped protect the reindeer, but also the indigenous peoples who depended on them for survival.

The M-M-R vaccine
MSD began distributing a combined measles-mumps-rubella (M-M-R) vaccine that was developed by Drs. Maurice Hilleman and Eugene B. Buynak. M-M-R was composed of three vaccines: ATTENUVAX, an updated version of MSD’s measles vaccine; MERUVAX, a rubella vaccine; and MUMPSVAX, MSD’s mumps vaccine.
First pneumonia vaccine was approved
PNEUMOVAX (pneumococcal vaccine polyvalent), MSD's pneumonia vaccine, was approved. Research and development of the vaccine was carried out under the direction of Dr. Maurice Hilleman.
FDA approved Mefoxin
MEFOXIN was indicated for the treatment of many infections caused by certain bacteria, including gram-positive and gram-negative pathogens.
VASOTEC was approved by the FDA
VASOTEC (enalapril), an angiotensin converting enzyme (ACE) inhibitor for treatment of high blood pressure and congestive heart failure, was approved by the FDA. VASOTEC became our first billion-dollar product in 1988.

Hepatitis B vaccine was approved by the FDA
Our recombinant hepatitis B vaccine, RECOMBIVAX HB [hepatitis b vaccine (recombinant)], was approved by the FDA, the first recombinant vaccine for human use. In 1989, we transferred RECOMBIVAX HB vaccine technology to China, where hepatitis B was the largest public health challenge in the country with an estimated 100 million carriers of the disease.

MSD introduced the first commercial statin
We introduced lovastatin, the first of the statin family of medicines to be approved by the FDA. It emerged from decades of study by scientists, including ours, around the world.

MSD committed to donate Mectizan - as much as needed for as long as needed - with the goal to eliminate river blindness.
In 1987, CEO Dr. Roy Vagelos announced our commitment to donate Mectizan – as much as needed for as long as needed – with the goal to help eliminate river blindness. In order to reach this goal, our leaders recognized that many organizations with unique skills would need to work together as a team. Thus, the Mectizan Donation Program (MDP) was created as a ground-breaking public-private partnership that becomes influential in the development of other drug donation programs.

CRIXIVAN (INDINAVIR SULFATE) approved by the FDA
CRIXIVAN (INDINAVIR SULFATE), for the treatment of HIV, was approved by the FDA after a review period of 42 days. Prior to the FDA approval in 1995, in conjunction with the U.S. Food and Drug Administration, patients and HIV advocacy groups, MSD decided to offer the Crixivan Program. Through this program, we made the drug available at no cost to selective patients before it was commercially available.

SINGULAIR approved by FDA
SINGULAIR (montelukast sodium) was approved by the FDA for the prevention and treatment of asthma. It was the product of nearly twenty years of research conducted at our discovery hub in Kirkland, Canada, into a new class of asthma medicines called leukotriene blockers.

JANUVIA (sitagliptin) approved by the FDA.
The FDA approved JANUVIA (sitagliptin), a DPP-4 inhibitor approved to treat Type 2 diabetes.

MSD’s GARDASIL approved by the FDA
The FDA approved GARDASIL [human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant], for the prevention of cervical cancer caused by certain HPV types.
In 2007, MSD committed to donating 3 million doses of GARDASIL over 5 years to support vaccination programs in the world's lowest-income nations.
Merger with Schering-Plough
MSD and Schering-Plough completed a merger and began combined operations. The purchase made the company the second largest pharmaceutical company in United States by revenue.


Global MSD for Mothers initiative began
In 2010, one woman died every two minutes during childbirth and pregnancy. Many of these deaths were preventable. In response, we launched MSD for Mothers, a global initiative with partners to improve the health and well-being of women before, during and after pregnancy and childbirth. As of 2019, the effort has reached more than nine million women globally in 48 countries.

BRAVECTO approved by the FDA for the MSD Animal Health division
The FDA approved BRAVECTO (fluralaner), the first chewable tablets for dogs to be shown to kill fleas and multiple tick species for 12 weeks in a single dose.
MSD received accelerated approval of KEYTRUDA (pembrolizumab)
The FDA approved KEYTRUDA (pembrolizumab), the first anti-PD-1 (programmed death receptor-1) therapy. It had previously received breakthrough therapy designation from the FDA.
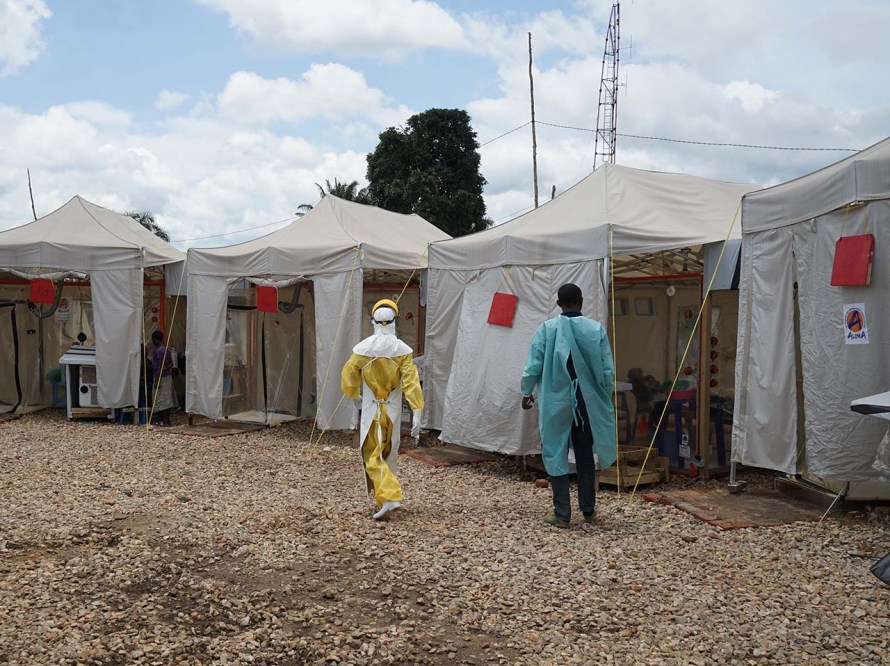
ERVEBO® approved by the FDA
From Guinea to the Democratic Republic of the Congo (DRC), the world was dealing with the largest and most complex Ebola outbreaks since the virus was first discovered in 1976. As the outbreaks remained a global health challenge, MSD scientists, along with numerous external collaborators from all sectors, remained at the forefront of the efforts to address this deadly disease.
MSD received FDA approval for ERVEBO® (Ebola Zaire Vaccine, Live) for the prevention of disease caused by Zaire ebolavirus in individuals 18 years of age and older.